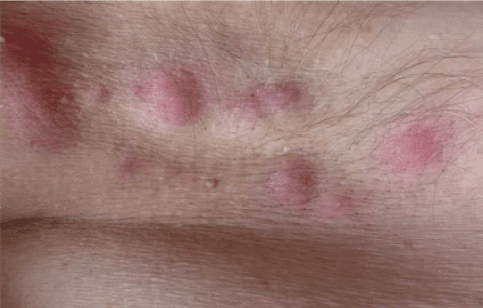
Hidradenitis Suppurativa – Adolescents and Adults
Adults and adolescents who have a diagnosis of moderate to severe hidradenitis suppurativa (HS) may qualify for this clinical research trial and receive investigational injectable medication or placebo.
- Age: ≥ 16 years old
- Diagnosis of HS ≥ 6 months
- HS lesions in ≥ 2 distinct anatomical areas and total lesion count ≥ 5
- Inadequate response or intolerance to a 12-week trial of oral antibiotics
Patients will be asked to visit the clinic about 19 times over approximately 52 weeks. There is no cost to participate, and participants will be reimbursed for their time and travel.
For further information on the study, please follow the link:
Trial ID: NCT06468228
Accreditations & Memberships





















